High-throughput genotyping of G6PD variants in heterozygous females
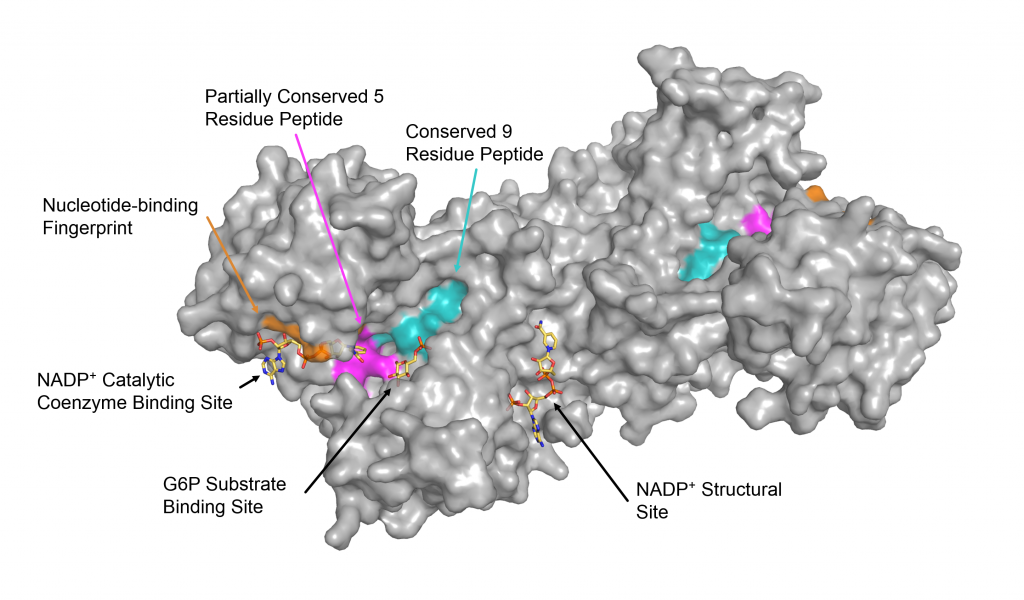
Glucose-6-phosphate dehydrogenase (G6PD) is the fist and rate-timing enzyme in the pentose phosphate pathway, catalyzing the conversion of glucose-6-phosphate (G6P) to 6-phosphogluconate and producing the reduced form of nicotinamide adenine dinucleotide phosphate (NADP-H), which plays important roles in the protection of cells against oxidative stress(3). G6PD is especially critical in red blood cells because it is the only source of reducing equivalents, G6PD deficiency is an inherited genetic disorder that is one of the most common enzymopathies in human, affecting over 400 million people worldwide (2). G6rD deficiency is caused by the mutation(s) in good gene which is located on the xq28 region, good gene is ~18 kb and comprises 13 exons and 12 introns. As an X-inked recessive hereditary disorder, clinical manifestations of G6PD deficiency are more prominent in male hemizygote and female homozygote, Heterozygous deficient females show variable phenotypes as a result of mosaic inactivation of X-chromosome. To date, more than 200 G6PD mutations have been identified and reported around the world. Most of G6pD variants reported are single point mutation, causing amino acid substitution (3). Some of G6PD variants have been characterized at the protein level and it was demonstrated that different point mutations result in different enzyme activity and stability (4, 5). Consequently, clinical manifestations caused by G6PD deficiency can be varied from asymptomatic to severe hemolysis and even death. Antimalarial drugs such as primaquine, tafenoquine, sulfonamides, and sulfones, have been reported to cause hemolytic anemia in G6PD deficient individuals (1), The mechanism underlying hemolysis is poorly understood. However, it is clearly demonstrated that drug-induced oxidative stress leads to red blood cell destruction. Accordingly, hemolytic risk in G6PD deficient individuals can cause medical problem to malaria treatment. Importantly, this is the obstacle to achieve the goal of malaria elimination. Plasmodium falciparum and P, vivox are the two main parasite species causing malaria. Infection with p, falciparum and P, vivax requires the administration of 8-aminoquinolines. Currently, primaquine and tafenoquine are the only licensed 8-aminoquinoline drugs that can prevent the relapse in P, vivox by clearing off hypnozoites. Primaquine is also the only available antimalarial drug that actively eliminates falciparum gametocytes, blocking transmission (6) Due to G6PD deficiency is widespread in malaria endemic area, the use of primaquine and tafenoquine is limited. Malaria patients with 30%-70% G6PD. activity can receive primaquine with dose-adjustment under medical supervision. However, tafenoquine is only prescribed to those with more than 70% G6PD activity. Clinical manifestations of drug-induced hemolysis is varied from self-limiting to severe hemolysis which cannot be compensated and is life-threatening (7). The degree of hemolytic response depends on the dose and duration of drug administration and G6PD status of patients. Nevertheless, the occurrence and severity of hemolysis in G6PD deficiency cannot be certainly predicted from the G6PD enzyme activity. As different mutations in said gene result in a range of protein abnormalities, some of G6PD variants with normal activity can developed acute hemolysis by triggers. In particular, it has raised a significant concern regarding hemolytic risk in heterozygous females in some of those who show enzyme activity similar to normal individuals. It was reported that a heterozygous female developed acute hemolytic anemia as server as hemizygous male when receiving 8-aminoquinoline drugs (8). Therefore, diagnosis of G6PD deficiency prior to treatment is required and recommended. Additionally, genotypic detection of G6PD deficiency, particularly in heterozygous females, will support the medical assessment of risks and benefits of the use of primaquine and tafenoquine in malaria patients with G6PD deficiency. Detection of G6PD deficiency includes phenotypic and genotypic tests. Considering the wide spectrum of clinical manifestations among G6PD deficient individuals, only phenotypic method might not be adequate for making medical decision for delivering primaquine and tafenoquine. G6PD variants that exhibit normal enzyme activity can develop severe hemolysis when they are exposed to triggers. In addition to the quantitative G6PD enzyme activity assay, supplemental genotypic test that reliable to identify hemizygous, homozygous, and heterozygous. G6PD deficiency is therefore necessary. Several G6PD genotyping techniques have been developed and described. Generally, G6PD genotypic methods are based on polymerase chain reaction (PCR) single nucleotide polymorphism (SNP) analysis and DNA sequencing, However, these techniques are either expensive or low throughput. in the present study, we aimed to develop a high-throughput genotypic test based on high-resolution melting (HRM) for diagnosis of G6PD deficiency. We expected that the developed method will increase the accuracy of detection of G6PD deficiency, especially in heterozygous females. This implementation will encourage a safe medication of primaquine and tafenoquine, accelerating the success of malaria elimination.


